Ergomed Websites and Interactive Tools
With a multidisciplinary team from Moriarti Design, we built both corporate and clinical research websites. For a better understanding of the company's services, I designed interactive tools and various infographics.
About the project
To enhance user experience and ensure alignment with the company's overarching branding guidelines, the goal was to refine the website's interface to optimize usability, intuitiveness, and visual coherence. By meticulously adapting branding guidelines to align with the overall corporate scheme and ideas, the idea was to effectively communicate Ergomed's expertise and commitment to excellence in clinical research and pharmacovigilance.
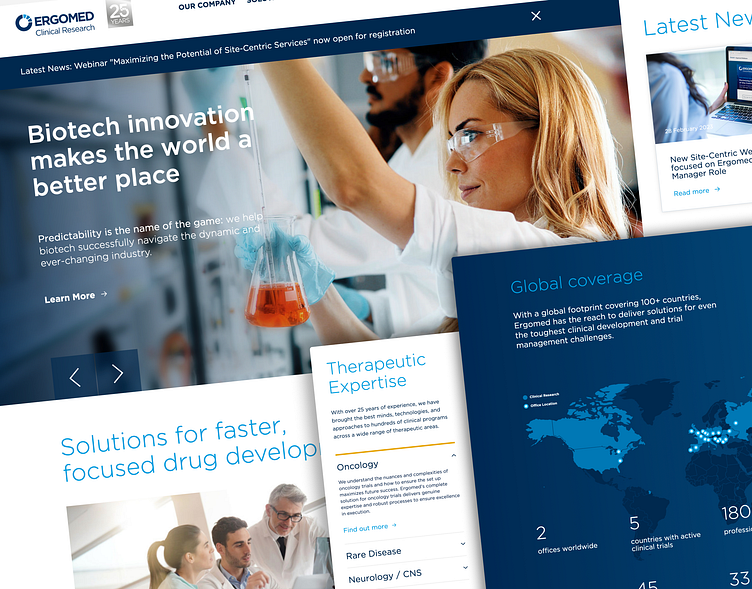
The website provides detailed information about Ergomed's services and expertise in clinical research, pharmacovigilance, and regulatory affairs. Ergomed highlights its experience and specialization in various therapeutic areas, including oncology, neurology, infectious diseases, rare diseases, and cardiovascular disorders. Each section provides insights into the company's expertise and track record in conducting clinical trials within these specific fields
On the Ergomed website, a primary focus was to showcase the company's extensive global presence and representation through numerous offices worldwide. This emphasis underscores Ergomed's stature as one of the leading providers of clinical research and pharmacovigilance services on a global scale.
Throughout the website, the strategic placement of information and visuals highlights the geographic reach of Ergomed, reinforcing the message of its widespread presence. Elements such as a global map displaying office locations, dedicated sections for regional offices, and testimonials from clients across different regions serve to underscore the company's global footprint.
Additionally, the website features content that emphasizes Ergomed CRO's ability to navigate diverse regulatory landscapes and cultural contexts, demonstrating its capacity to deliver tailored solutions to clients worldwide. Whether through case studies showcasing successful collaborations in various regions or testimonials highlighting the company's responsiveness and adaptability, the website effectively communicates Ergomed's commitment to providing localized expertise with a global perspective.
The content
The Solutions section serves as the focal point and pinnacle of Ergomed's website, encapsulating the company's comprehensive offerings, expertise, and value proposition in clinical research and pharmacovigilance. With its comprehensive overview, detailed descriptions, and clear calls to action, this section effectively communicates the company's value proposition.
In response to the challenge of effectively communicating Ergomed comprehensive involvement in the entire clinical development process, an interactive graphic was introduced in this section. The interactive graphic offers a visually engaging representation of the clinical development lifecycle, highlighting key milestones, processes, and services provided by Ergomed at each stage.
The Information and Resources section serves as a repository of informative content designed to provide valuable insights and resources for visitors interested in clinical research and pharmacovigilance. Case Studies are detailed narratives that highlight specific projects or collaborations undertaken by Ergomed. White Papers are in-depth reports or articles that delve into specific topics or trends relevant to the clinical research and pharmacovigilance industry. The Articles section may feature blog posts, news updates, or opinion pieces authored by experts within Ergomed or guest contributors. These articles cover a wide range of topics, including industry trends, regulatory updates, best practices, and thought leadership insights, providing readers with valuable perspectives and knowledge.
The Resources section also features a diverse range of content formats, including webinars and podcasts. These multimedia resources provide additional avenues for visitors to access valuable insights, expert perspectives, and industry trends related to clinical research and pharmacovigilance.
Services and interactive tools
The Services section provides a comprehensive overview of the company's extensive capabilities and expertise in clinical research and pharmacovigilance. For this purpose, a few interactive tools have been developed to augment the content and provide enhanced support to visitors navigating through the services offered.
For example, in the Regulatory Affairs Services subsection, an interactive tool has been implemented to offer users a dynamic and engaging way to explore regulatory strategy development. Through this tool, users can interactively navigate through different aspects of regulatory affairs, such as submission management and post-approval support, gaining valuable insights into Ergomed CRO's expertise and approach in this critical area.
In the Study Start-Up and Activation subsection, an interactive tool has been developed to guide users through the intricate process of initiating and activating clinical studies. The tool offers a step-by-step walkthrough of the study start-up and activation process, breaking down complex procedures into digestible segments. Users can interactively explore each stage of the process, from protocol development and regulatory submissions to site selection and study initiation.
These interactive tools serve as valuable resources for visitors seeking to deepen their understanding of the services offered, ultimately empowering them to make informed decisions and engage more meaningfully.
Additional content
The main website sections are intricately interconnected with the Resources section, leveraging whitepapers and case studies to enrich and support the content across various thematic areas, like in the case of the Therapeutic Expertise section. This approach enhances credibility, fosters engagement, and empowers visitors with actionable insights to inform their decision-making processes.